38 fda approved drug labels
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (h) Medication Guide means FDA-approved patient labeling conforming to the specifications set forth in this part and other applicable regulations. (i) Packer means a person who packages a drug... Alnylam Announces FDA Approval of Supplemental New Drug Application for ... cambridge, mass., october 07, 2022 -- ( business wire )-- alnylam pharmaceuticals, inc. (nasdaq: alny), the leading rnai therapeutics company, announced today that the u.s. food and drug...
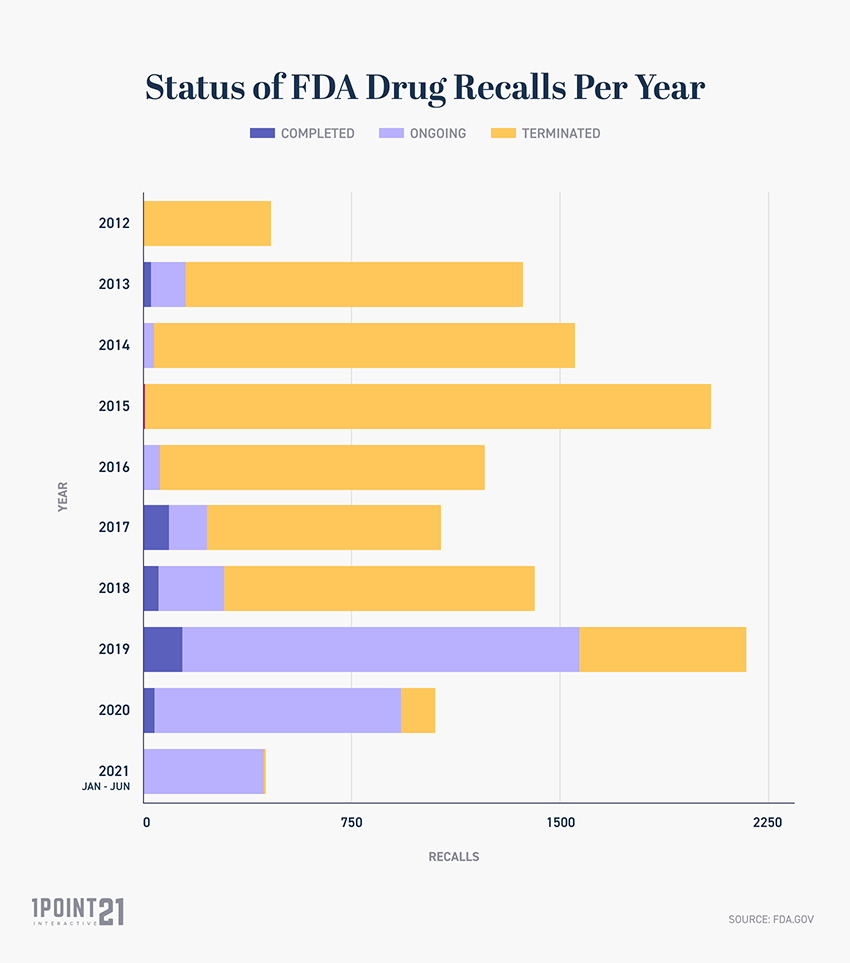
FDA revises labels of SGLT2 inhibitors for diabetes to include warnings ... [12-4-2015] A U.S. Food and Drug Administration (FDA) safety review has resulted in adding warnings to the labels of a specific class of type 2 diabetes medicines called sodium-glucose...

Fda approved drug labels
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration The information on this page is current as of Mar 29, 2022. For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 312.6 Labeling of an investigational new drug. (a) The immediate package of an investigational new drug intended for human use shall bear a label with the statement "Caution ... Vaccine Labels: FDA Approved Vaccine Information from VxLabels.com As the leading independent provider of trustworthy vaccine information, our database comes directly from the FDA's central repository of drug labels and package inserts under the Structured Product Labeling standard. VxLabels.com provides the full vaccine subset of the FDA's repository. Alnylam Announces FDA Approval of Supplemental New Drug Application for ... CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Alnylam Pharmaceuticals, Inc. (Nasdaq: ALNY), the leading RNAi therapeutics company, announced today that the U.S. Food and Drug Administration (FDA) approved a label expansion for OXLUMO ® (lumasiran), an RNAi therapeutic administered via subcutaneous injection, now indicated for the treatment of primary hyperoxaluria type 1 (PH1) to lower urinary oxalate ...
Fda approved drug labels. FDA Approves AMX0035 (Relyvrio) for ALS | Everyday Health On September 29, 2022, the U.S. Food and Drug Administration (FDA) approved AMX0035 (Relyvrio) for the treatment of amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease. The ... Doctors urge U.S. FDA to add miscarriage management to abortion pill label Obstetricians, gynecologists, other medical professionals, and abortion rights advocates petitioned the U.S. Food and Drug Administration (FDA) on Tuesday to urge Danco Laboratories to seek ... Labeling for CBER-Regulated Products | FDA - U.S. Food and Drug ... For prescription brand-name drugs, Drugs@FDA typically includes the most recent labeling approved by the FDA (for example, Prescribing Information and FDA-approved patient labeling when available),... CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (1) Prescription drug labeling described in § 201.100 (d) must contain the specific information required under § 201.57 (a), (b), and (c) under the following headings and subheadings and in the...
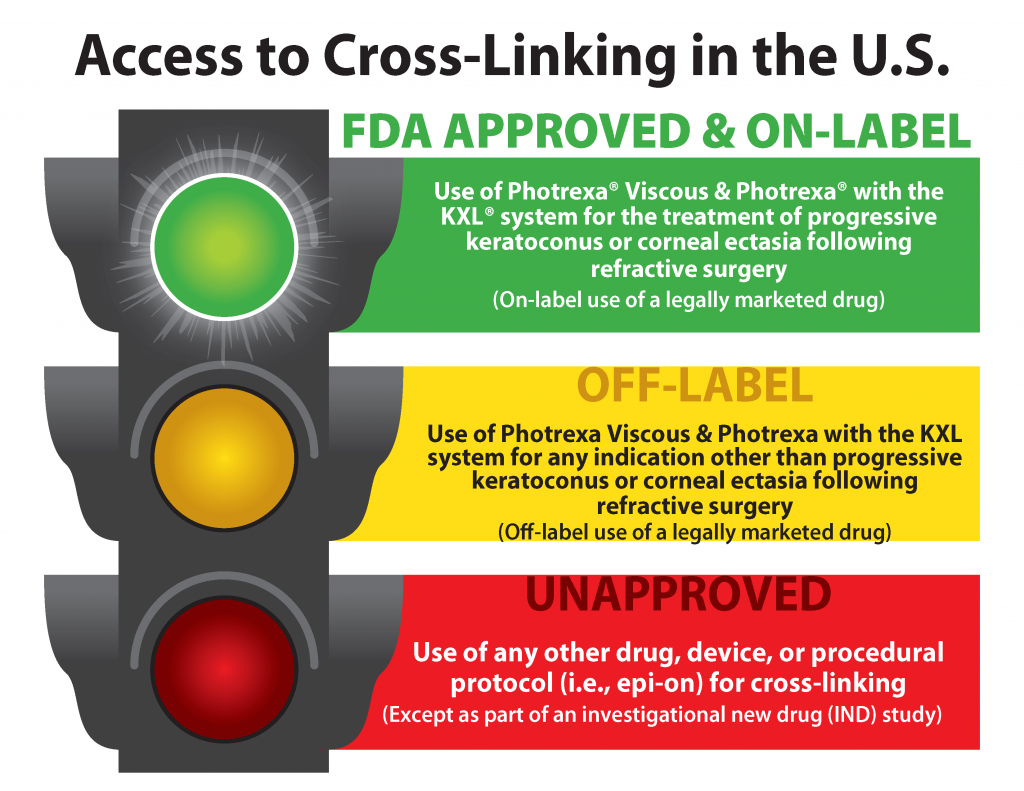
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (6) The requirement in § 201.80 (f) (2) to reprint any FDA-approved patient labeling at the end of prescription drug labeling or accompany the prescription drug labeling must be implemented no... FDA approval of supplemental new drug application for Oxlumo in ... The supplemental New Drug Application also included results from the open-label extensions of the ILLUMINATE-A and ILLUMINATE-B Phase III studies of pediatric and adult patients with PH1. The label has correspondingly been updated to highlight the maintenance of sustained reductions in UOx through Month 24 and Month 12, respectively. Drugs and Biologicals, Coverage of, for Label and Off-Label Uses Coverage Indications, Limitations, and/or Medical Necessity. Abstract: An off-label/unlabeled use of a drug is defined as a use for a non-FDA approved indication, that is, one that is not listed on the drug's official label/prescribing information. An indication is defined as a diagnosis, illness, injury, syndrome, condition, or other clinical ... Is It Really 'FDA Approved'? - U.S. Food and Drug Administration New drugs and biological products for people must be FDA approved before they are marketed in interstate commerce. This means that a company must demonstrate that its drug or biological product is...
› drugs › drug-safety-and-availabilityFDA Drug Safety Communication: FDA cautions about using ... [03-03-2015] The U.S. Food and Drug Administration (FDA) cautions that prescription testosterone products are approved only for men who have low testosterone levels caused by certain medical ... FDA Approves Label Extension for Evrysdi for Infants with Spinal ... SOUTH PLAINFIELD, N.J., May 31, 2022 /PRNewswire/ -- PTC Therapeutics, Inc. (NASDAQ: PTCT) today announced that the U.S. Food and Drug Administration (FDA) has approved a label extension for Evrysdi ( risdiplam) to include infants under 2 months old with spinal muscular atrophy (SMA). FDALabel: Full-Text Search of Drug Product Labeling | FDA The following table lists the count of several common labeling types in FDALabel. * Includes Human OTC drugs approved for marketing through a New Drug Application (NDA), Abbreviated New Drug... › drugs › science-and-research-drugsTable of Pharmacogenomic Biomarkers in Drug Labeling | FDA Aug 11, 2022 · The table below lists therapeutic products from Drugs@FDA with pharmacogenomic information found in the drug labeling. The labeling for some, but not all, of the products includes specific actions ...
› drugs › drug-safety-and-availabilityFDA revises labels of SGLT2 inhibitors for diabetes to ... Mar 15, 2022 · A U.S. Food and Drug Administration (FDA) safety review has resulted in adding warnings to the labels of a specific class of type 2 diabetes medicines called sodium-glucose cotransporter-2 (SGLT2 ...
› food › food-additives-petitionsColor Additives Questions and Answers for Consumers | FDA There are nine certified color additives approved by the FDA for use in food: FD&C Blue No. 1 Confections, beverages, cereals, frozen dairy desserts, popsicles, frostings & icings
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Any FDA-approved patient labeling must be referenced in this section and the full text of such patient labeling must be reprinted immediately following this section or, alternatively, accompany the prescription drug labeling. Any FDA-approved patient labeling printed immediately following this section or accompanying the labeling is subject to ...
› drugs › drug-safety-and-availabilityFDA requiring Boxed Warning updated to improve safe use of ... Oct 02, 2020 · Chlordiazepoxide was the first benzodiazepine approved in 1960, and FDA approved many subsequent medicines in this class in the 1960s and 1970s (see List of Benzodiazepines).
Drug Approvals and Databases | FDA Drug and Biologic Approval and IND Activity Reports. Drug Trials Snapshots. Oncology (Cancer) / Hematologic Malignancies Approval Notifications. FDALabel. FDA Online Label Repository. FDA's ...
FDALabel: Full-Text Search of Drug Product Labeling | FDA The following table lists the count of several common labeling types in FDALabel. * Includes Human OTC drugs approved for marketing through a New Drug Application (NDA), Abbreviated New Drug...
FDA's Labeling Resources for Human Prescription Drugs | FDA Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing Information,...
› food › buy-store-serve-safe-foodSelecting and Serving Fresh and Frozen Seafood Safely | FDA Feb 17, 2022 · Look for the label: Look for tags on sacks or containers of live shellfish (in the shell) and labels on containers or packages of shucked shellfish. These tags and labels contain specific ...
FDA List of Authorized Generic Drugs | FDA - U.S. Food and Drug ... To obtain approval of a generic drug, a company must submit an Abbreviated New Drug Application (ANDA) to FDA and prove that its product is the same as the brand-name drug in the ways described...
› drugs › drug-safety-and-availabilityFDA updates on hand sanitizers consumers should not use Sep 23, 2022 · FDA-tested product; contains benzene; FDA recommended the company recall Lot 2004718 (Expiration 04/2023) on 12/27/2021; FDA expanded recall on 2/25/2022 to include all hand sanitizer drug ...
Over the Counter Medications: FDA Approved Information from OTCLabels.com DayTime Cold and Flu Non Drowsy (ACETAMINOHPEN, DEXTROMETHORPHAN HBr, PHENYLEPHRINE HCl) Temporarily relieves common cold and flu symptoms minor aches and pains, headache, sore throat, nasal congestion, fever, cough due to minor throat and bronchial irritation. Warnings:… Cardinal Health (Leader) 70000 30 September 2022
Alnylam Announces FDA Approval of Supplemental New Drug Application for ... CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Alnylam Pharmaceuticals, Inc. (Nasdaq: ALNY), the leading RNAi therapeutics company, announced today that the U.S. Food and Drug Administration (FDA) approved a label expansion for OXLUMO ® (lumasiran), an RNAi therapeutic administered via subcutaneous injection, now indicated for the treatment of primary hyperoxaluria type 1 (PH1) to lower urinary oxalate ...
Vaccine Labels: FDA Approved Vaccine Information from VxLabels.com As the leading independent provider of trustworthy vaccine information, our database comes directly from the FDA's central repository of drug labels and package inserts under the Structured Product Labeling standard. VxLabels.com provides the full vaccine subset of the FDA's repository.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration The information on this page is current as of Mar 29, 2022. For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 312.6 Labeling of an investigational new drug. (a) The immediate package of an investigational new drug intended for human use shall bear a label with the statement "Caution ...







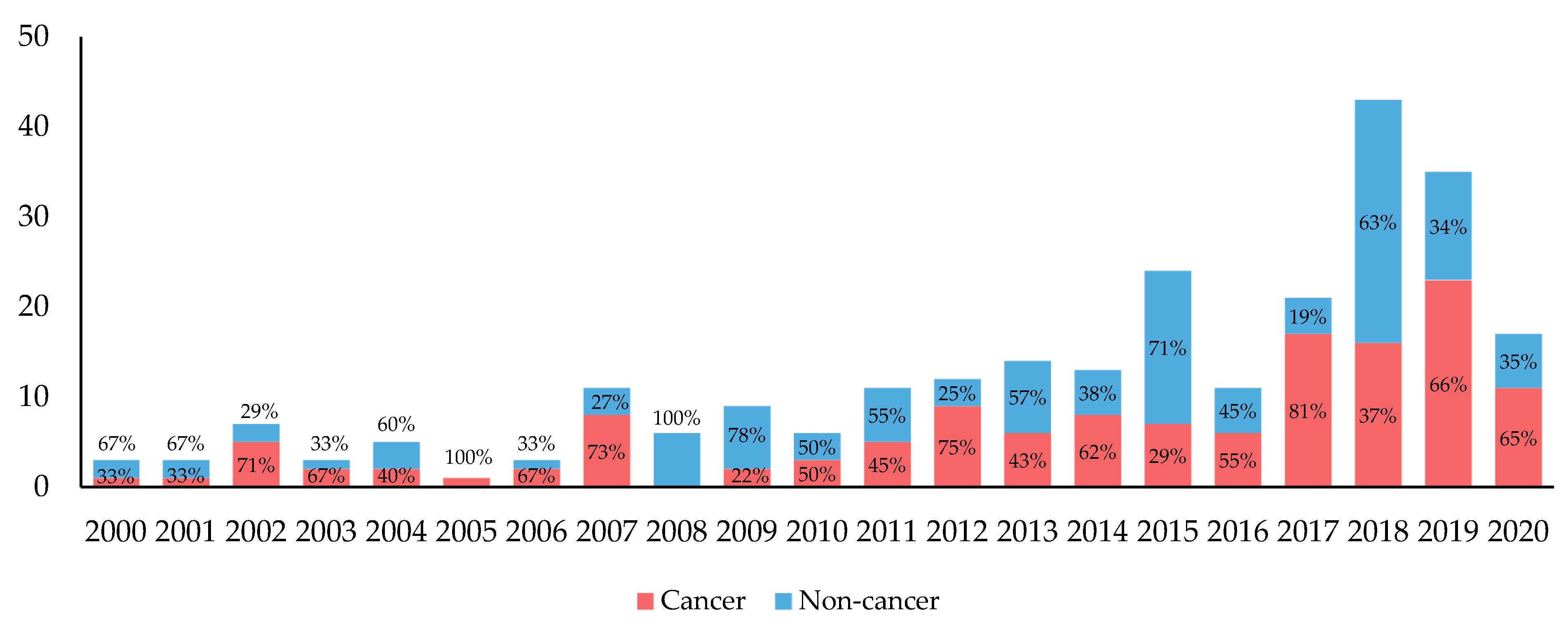

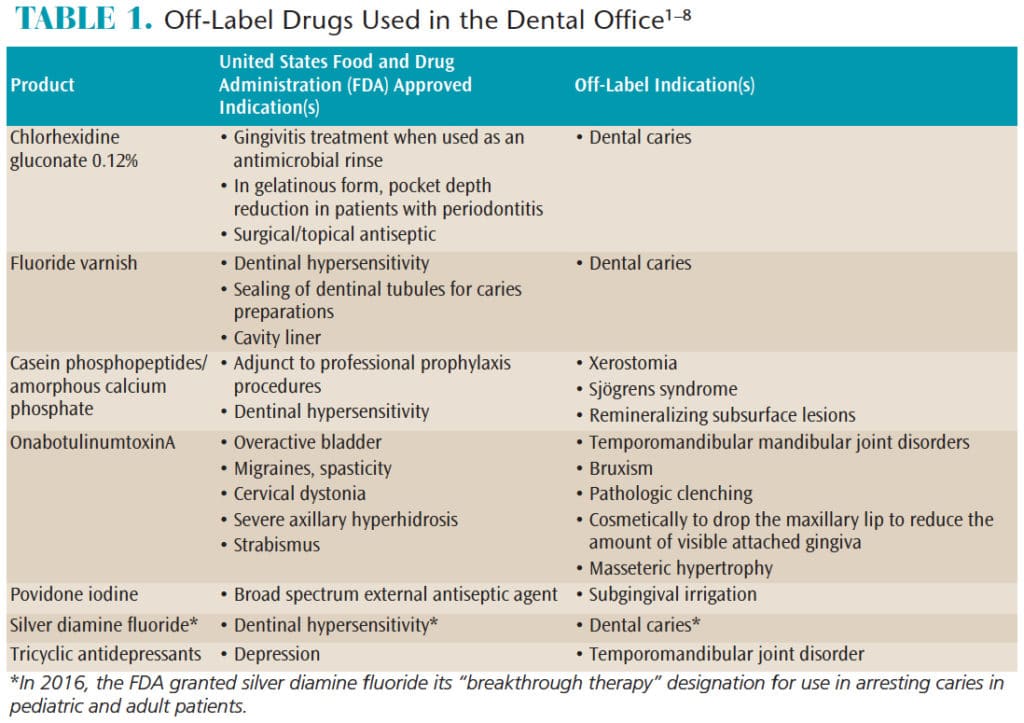
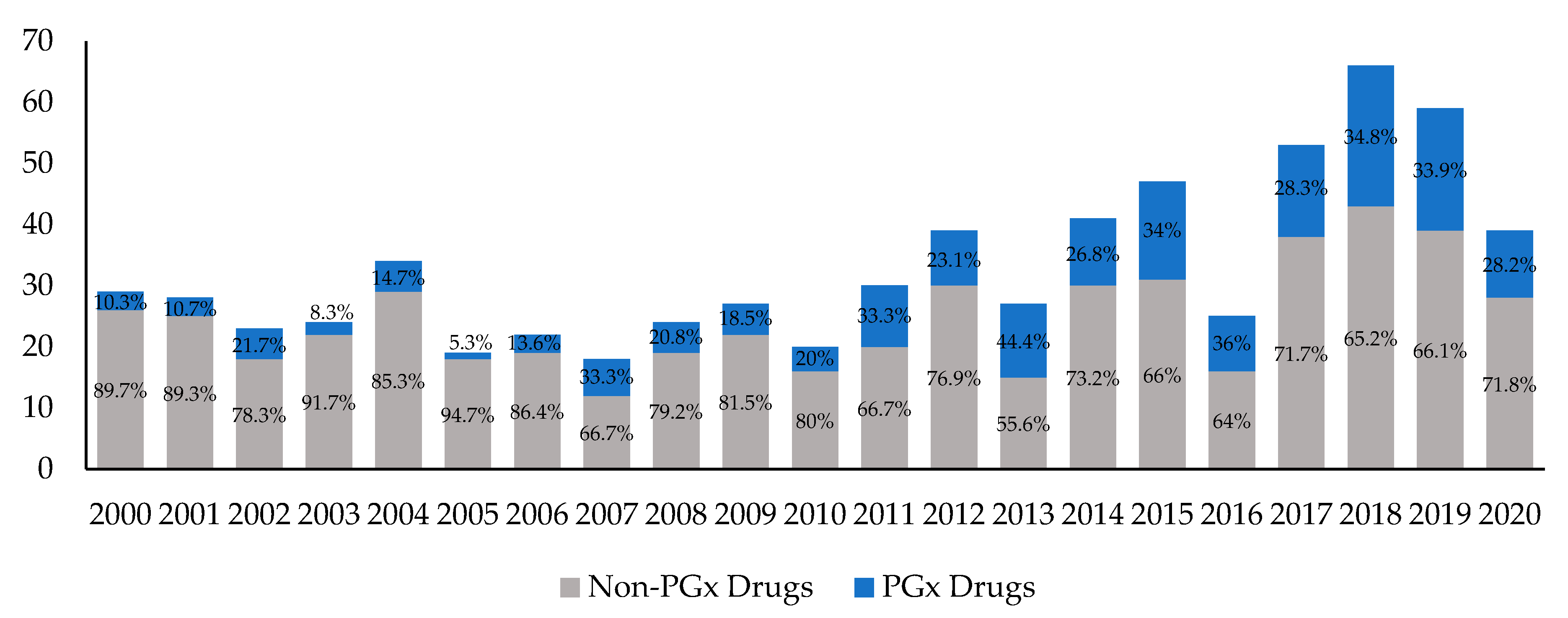







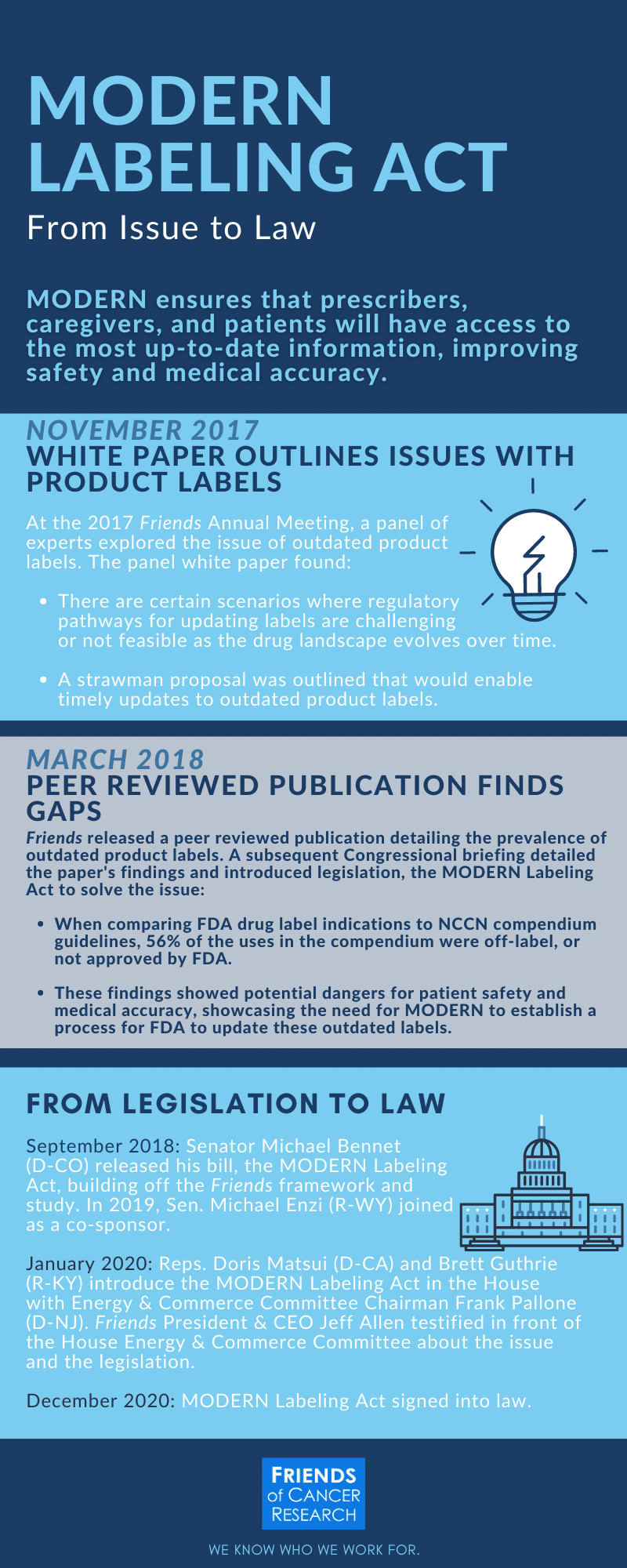


:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/4097058/flibanserin%20with%20DH%20v4%20(post-label)%20(1).jpg)

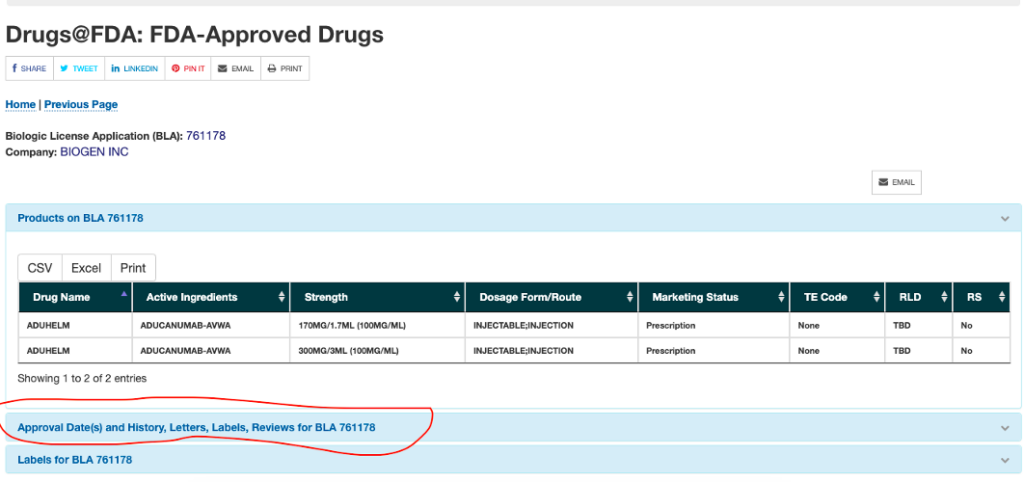

Post a Comment for "38 fda approved drug labels"