45 phase labels in chemical equations
Balancing Chemical Equations | CK-12 Foundation Ordinary Differential Equations · DifferentialEquations.jl - SciML AutoVern7(Rodas5()) handles both stiff and non-stiff equations in a way that's efficient for high accuracy. Tsit5() for standard non-stiff. This is the first algorithm to try in most cases. BS3() for fast low accuracy non-stiff. Vern7() for high accuracy non-stiff. Rodas4() or Rodas5() for small stiff equations with Julia-defined types, events ...
Definition of dissolve and the state label (aq) in chemical equations C O X 2 ( a q) would imply dissolved carbon dioxide in water (i.e.,to our eyes it is a single phase, just like an unopened bottle of coke). B r X 2 ( a q) would imply homogeneous solution of liquid bromine in water. N a C l ( a q): Salt dissolved in water. A colloid is a heterogeneous phase.

Phase labels in chemical equations
Chapter 4 Quantities of reactants and products Answered: Complete and balance the following… | bartleby Solution for Complete and balance the following molecular equations (indicate the relevant phase labels): LICI (s) + HSO4 (aq) → NH. (aq) + OH(aq) → AgNOs (aq)… Skip to main content. close. Start your trial now! First week only $4.99! arrow_forward. Literature guides Concept explainers Writing guide Popular textbooks Popular high school textbooks Popular Q&A … End-of-Chapter Material - Lardbucket.org Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl2(g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2O2(g) → H2O (ℓ) should not be considered a proper chemical equation.
Phase labels in chemical equations. 3 Steps for Balancing Chemical Equations - ThoughtCo A chemical equation describes what happens in a chemical reaction.The equation identifies the reactants (starting materials) and products (resulting substances), the formulas of the participants, the phases of the participants (solid, liquid, gas), the direction of the chemical reaction, and the amount of each substance. Chemical equations are balanced for mass and charge, meaning the number ... Ch. 1 Exercises - Chemistry 2e | OpenStax 4.1 Writing and Balancing Chemical Equations; 4.2 Classifying Chemical Reactions; 4.3 Reaction Stoichiometry; 4.4 Reaction Yields; 4.5 Quantitative Chemical Analysis; Key Terms; Key Equations; Summary; Exercises; 5 Thermochemistry. Introduction; 5.1 Energy Basics; 5.2 Calorimetry; 5.3 Enthalpy; Key Terms; Key Equations; Summary; Exercises; 6 Electronic … openstax.org › books › chemistry-2eCh. 1 Exercises - Chemistry 2e | OpenStax Classify the six underlined properties in the following paragraph as chemical or physical: Fluorine is a pale yellow gas that reacts with most substances . The free element melts at −220 °C and boils at −188 °C . End-of-Chapter Material - Introductory Chemistry- 1st Canadian Edition Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation.
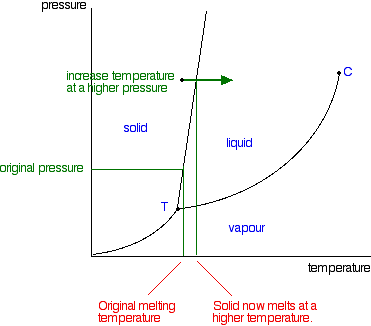
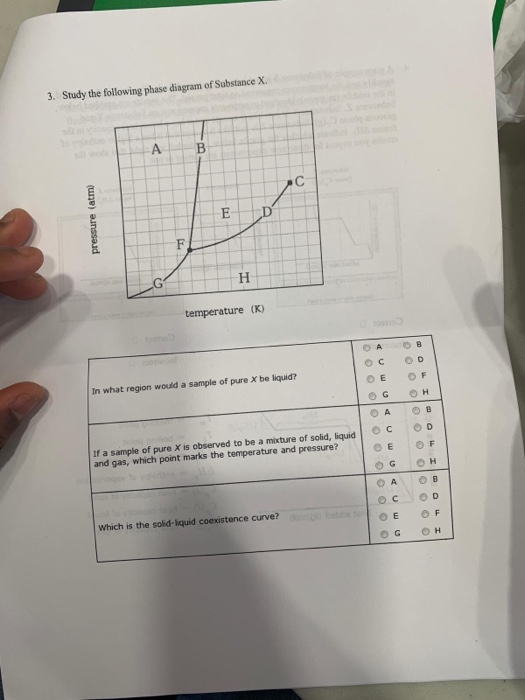
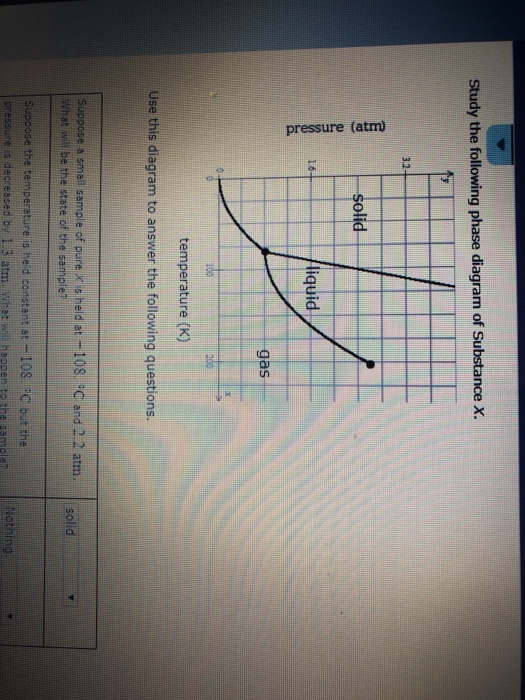
5.2 Chemical Equations - Lumen Learning It is not uncommon to include a phase label with each formula— (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for a substance dissolved in water, also known as an aqueous solution. If we included phase labels for the reactants and products, under normal environmental conditions, the reaction would be as follows: H2(g) + O2(g) → H2O (ℓ) Note Phase Diagrams - Chemistry - University of Hawaiʻi (a) On the phase diagram, label the gas and liquid regions. (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase. (c) If graphite at normal conditions is heated to 2500 K while the pressure is increased to 10 10 Pa, it is converted into diamond. Label the diamond phase. Journal of Chemical Education | Vol 99, No 2 - ACS Publications 08/02/2022 · Electroreduction of CO2 to useful chemicals is a rapidly developing field of study and one of the foundational technologies for a carbon-neutral future. Integrating laboratory practice on this topic in undergraduate chemistry curricula is an excellent opportunity to improve student exposure and basic training in synthetic electrochemistry. In the article, the authors describe a … 5.2 Chemical Equations | The Basics of General, Organic, and Biological ... It is common to include a phase label with each formula— (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for a substance dissolved in water, also known as an aqueous solution. Including phase labels for the reactants and products, under normal environmental conditions, the reaction would be as follows: H2(g) + O2(g) → H2O (ℓ) Note
Solved 1. Write balanced chemical equations using proper | Chegg.com 1. Write balanced chemical equations using proper phase labels for these reactions: (you may also handwrite in the equations) a) Iron metal with oxygen gas to produce solid iron (III) oxide, also known as rust. diffeq.sciml.ai › stable › tutorialsOrdinary Differential Equations · DifferentialEquations.jl AutoVern7(Rodas5()) handles both stiff and non-stiff equations in a way that's efficient for high accuracy. Tsit5() for standard non-stiff. This is the first algorithm to try in most cases. BS3() for fast low accuracy non-stiff. Vern7() for high accuracy non-stiff. Rodas4() or Rodas5() for small stiff equations with Julia-defined types, events ... State symbols and phase changes | StudyPug The phase can affect how reactive a substance is, but changing phase (a physical change) is not the same as changing the substance (a chemical change). A fully detailed chemical equation will show the state (or phase) of matter that the atoms or molecules are in. These states are: Solid, given the symbol (s) Liquid, given the symbol (l) Droplet-based microfluidics - Wikipedia Droplet-based microfluidics manipulate discrete volumes of fluids in immiscible phases with low Reynolds number and laminar flow regimes. Interest in droplet-based microfluidics systems has been growing substantially in past decades. Microdroplets offer the feasibility of handling miniature volumes (μl to fl) of fluids conveniently, provide better mixing, encapsulation, sorting, …
Phase Diagram | Explanation, Definition, Summary & Facts A phase has a complete distinct physical and chemical properties, whereas two phases are separated by a phase boundary. A phase diagram is a graphical representation of the substance phases, consists of the curved lines and the space between the two lines represent a specific phase of the matter at given pressure and temperature, whereas any ...
The Chemical Equation - GitHub Pages In chemical equations, the number of atoms of each element in the reactants must be the same as the number of atoms of each element in the products. ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as ...
› chemistry-solversChemistry Problem Solver Online | Chemistry Homework Help ... If those strange equations a chemistry teacher writes on a chalkboard drive you crazy, there’s no need in torturing yourself. Thanks to the digital and technological progress, you can find a chemistry problem solver for any occasion. Here are some you can use. Chem Wiz; pH Calculator; Balancing Chemical Equations; Mole Calculator
en.wikipedia.org › wiki › Scientific_lawScientific law - Wikipedia Scientific laws or laws of science are statements, based on repeated experiments or observations, that describe or predict a range of natural phenomena. The term law has diverse usage in many cases (approximate, accurate, broad, or narrow) across all fields of natural science (physics, chemistry, astronomy, geoscience, biology).
chemical equation | Yeah Chemistry Formulas of the reactants appear on the left side of the equation; those of products are written on the right. In many cases it is useful to indicate the state or phases of the substance in an equation.You use the following phase labels: (g) = gas , (l) = liquid , (s) = solid , (aq) = water solution
Tinker, Taylor, Builder, Nexus (Worm/Eclipse Phase ... - SpaceBattles 10/03/2022 · Recent threadmarks Chapter 3-3 - Format, part 3 - In which the shit hits the fan. New Chapter 3-2 - Format, part 2 - In which Taylor finally meets her heroes. New Chapter 3-1 - Format, part 1 - wherein Taylor gets a move on New Interlude 3 - Protectorate & Undersiders - wherein several people try to figure out what the fuck just happened. New Chapter 2-10 - POST, part 10 …
Chemical Equations - GitHub Pages If we included phase labels for the reactants and products, under normal environmental conditions, the reaction would be as follows: H 2 (g) + O 2 (g) → H 2 O(ℓ) Note. ... To make this chemical equation conform to the law of conservation of matter, we must revise the amounts of the reactants and the products as necessary to get the same ...
The Chemical Equation | Introductory Chemistry - Lumen Learning Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, 2 NaHCO3(s) →200°C Na2CO3(s) + CO2(g) + H2O (ℓ) Key Takeaways
The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Write and balance the chemical equation that represents nitrogen and hydrogen reacting to produce ammonia, NH 3. Answer N 2 + 3H 2 → 2NH 3 Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water).
Chemistry Problem Solver Online - Online Homework Help for … When you need to calculate molar mass or to balance a chemical equation, you can count on the chemistry problem solver to help you out. This tool is quite comprehensive. It is useful for any student regardless of the type and level of the chemistry course which he or she is taking. If you find things difficult to understand, the problem solver will help you with the learning process. If …
How to Balance: N2 + H2 = NH3 (Synthesis of Ammonia) - YouTube
End-of-Chapter Material | Introductory Chemistry - Lumen Learning Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation.
Solved Part 7: Aqueous Chemistry...Ionic Equations 7. In | Chegg.com In many aqueous chemical reactions, there are ions that are not involved in the chemical change but serve to deliver the ions that are involved. hen a compound in a chemical equation has an aqueous label, and is ionic, it consists of the constituent ions separated in solution. We represent the ions in a complete This question hasn't been solved yet
Phase Definition and Examples - ThoughtCo A phase of matter is characterized by having relatively uniform chemical and physical properties. Phases are different from states of matter. The states of matter (e.g., liquid, solid, gas) are phases, but matter can exist in different phases yet remain in the same state of matter. For example, liquid mixtures can exist in multiple phases, such ...
Solved 1. Write a balanced thermochemical equation with | Chegg.com Question: 1. Write a balanced thermochemical equation with phase labels for the Haber process with the heat energy as part of the equation. (3 pts) 2. What is the theoretical yield of ammonia (in grams) if 16.55 grams of nitrogen gas and 10.15 grams of hydrogen gas are allowed to react? (9 pts) 3.
End-of-Chapter Material - Introductory Chemistry - 1st Canadian Edition Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl 2 (g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H 2 (g) + ½O 2 (g) → H 2 O (ℓ) should not be considered a proper chemical equation.
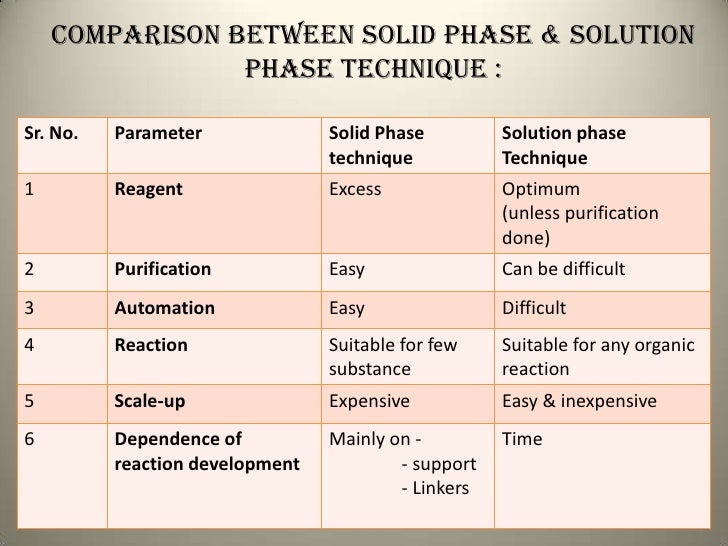


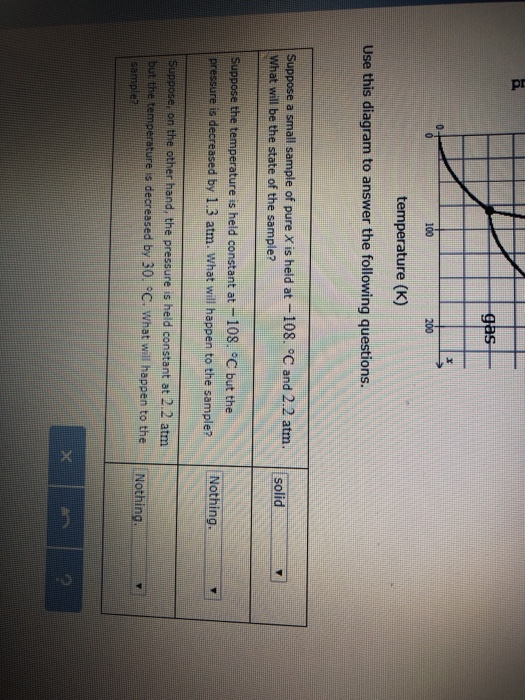



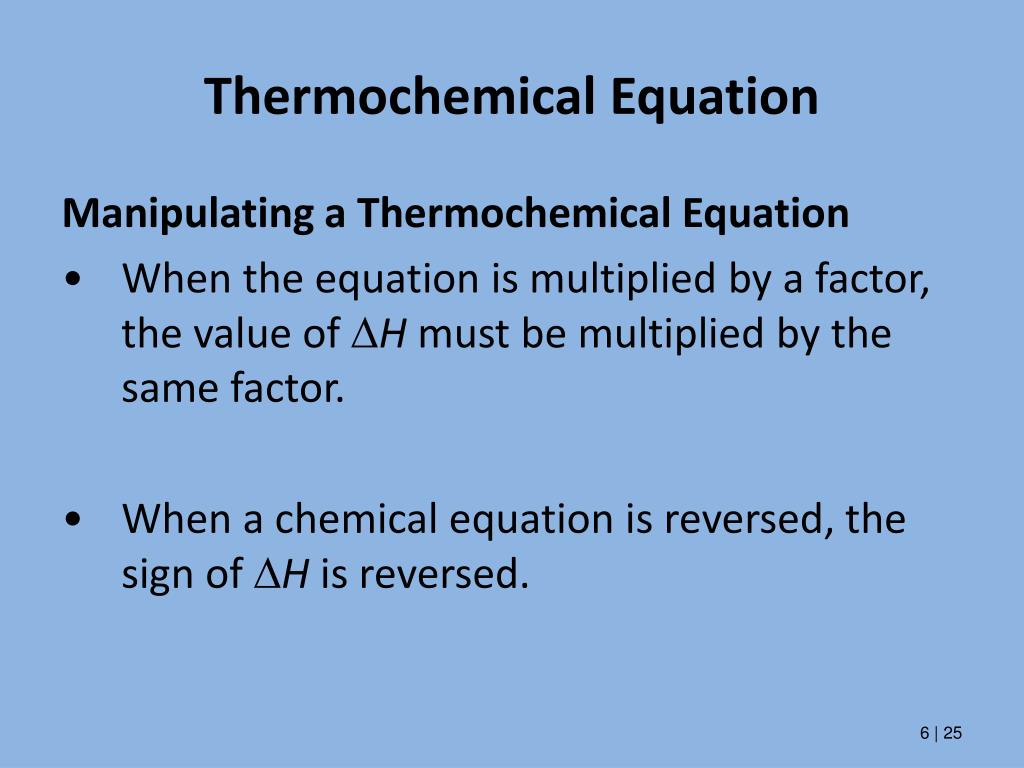
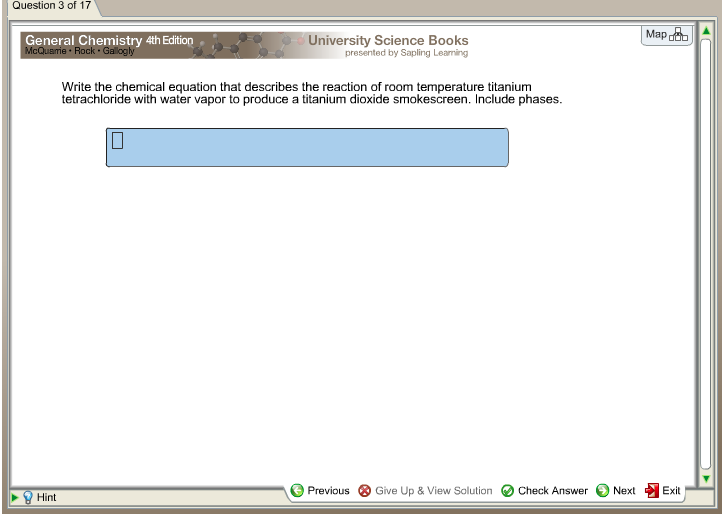


Post a Comment for "45 phase labels in chemical equations"